Anaesthetic machine preparation for MH-susceptible patients and MH crisis management.
-
Preparation of any anaesthesia workstation in less than 90 seconds for a known MH patient – Saves time/money as flushing can take 20 minutes normally
-
Allows Vapour-Free anaesthesia machine/s to be used elsewhere, instead of being kept on stand-by and un-utilised – Saves money and time by not having to secure the machine
-
Can be used for a crisis situation case where MH was not previously known – can help save lives
| CODE | DESCRIPTION | UNIT |
|---|---|---|
| 101AU | Pair of Vapor-Clean Filters | 8 pairs/pack |
|
|
Prevent a Malignant Hyperthermia Crisis
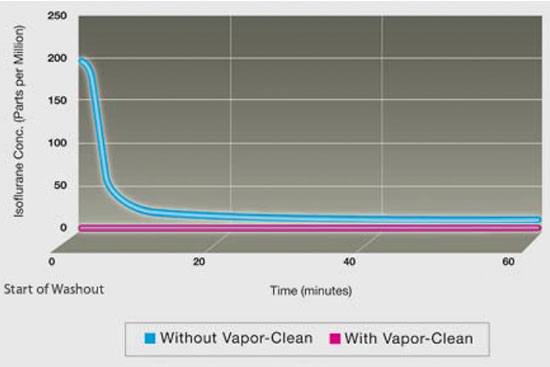
Newer anaesthetic gas machines contain plastic and elastomeric components that absorb volatile anaesthetics and then release residual vapour during subsequent anaesthetic procedures. The anaesthetic gas machine requires high flows and a lengthy time period to remove most of the vapour before the machine can be used for a patient that cannot tolerate breathing trace amounts of volatile anaesthetic vapour. Furthermore, the Vapor-Clean filters are MRI safe (the same instructions for use are applicable for regular theatre use and MRI use).
The Vapor-Clean filters absorb the trace amounts of anaesthetic vapour (isoflurane, sevoflurane and desflurane) so that anaesthetic vapours do not reach the patient. Placement of the Vapor-Clean filter canisters on the anaesthetic machine allows the machine to be immediately vapour-free (less than 5 parts per million of vapour).
Key features:
- Compatible with most Anaesthesia Machines
- Ready in Under 90 Seconds
- Two-Year Minimum Shelf Life
- Suitable for use with MRI
- Negligible Additional Breathing Circuit Resistance
- No Need to Remove CO2 Absorbent
- Compatible with Both Standard Two-Limb and Coaxial Breathing Circuits