Sterile Tourniquets Prevent Surgical Site Infection
HemaClear® is a sterile exsanguination tourniquet that is used during limb surgery to provide a bloodless surgical field. It replaces the pneumatic tourniquet and the Esmarch bandage that have been in routine use for over 100 years. There is an increased risk of Surgical Site Infection (SSI) with non-sterile pneumatic tourniquets, whereas the sterile HemaClear® range is 100% safe. Below is an examination of non-sterile tourniquets at St Peter’s Hospital in London, further perpetuating the notion that sterile tourniquets prevent surgical site infection.
The effect of sterile versus non-sterile tourniquets on microbiological colonisation in lower limb surgery.
Author information
- 1
- St Peter’s Hospital, Chertsey, UK.
Abstract
INTRODUCTION:
Surgical tourniquets are commonplace in lower limb surgery. Several studies have shown that tourniquets can be a potential source of microbial contamination but have not compared the use of sterile versus non-sterile tourniquets in the same procedures.
METHODS:
Patients undergoing elective orthopaedic lower limb surgery were randomised prospectively to use of non-sterile pneumatic tourniquet or sterile elastic exsanguination tourniquet (S-MART™, OHK Medical Devices, haifa, Israel). Samples were taken from the ties of the non-sterile tourniquet prior to surgery and from the sterile tourniquets at the end of the operation in a sterile fashion. These were then sealed in universal containers and immediately analysed by the microbiology department on agar plates, cultured and incubated.
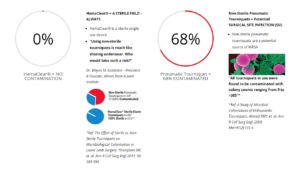
RESULTS:
Thirty-four non-sterile tourniquets were sampled prior to surgical application, twenty-three of which were contaminated with several different organisms including coagulase-negative Staphylococcus spp, Staphylococcus aureus, Sphingomonas paucimobilis, Bacillus spp, and coliforms. Thirty-six sterile tourniquets were used, with no associated contamination.
CONCLUSIONS:
There was significant contamination of 68% of orthopaedic surgical tourniquets. These are used regularly in procedures involving the placement of prosthesis and metalwork, and can act as a potential source of infection. We recommend the use of sterile single-use disposable tourniquets where possible. The availability of an alternative should now set the new standard of care and we recommend adopting this as a current NICE guideline for control of surgical site infection.